FDA Approves First Drug Treatment for Obstructive Sleep Apnea, Marking New Era in Sleep Medicine

In a groundbreaking development for sleep medicine, the U.S. Food and Drug Administration has approved Zepbound (tirzepatide) as the first-ever drug treatment for obstructive sleep apnea (OSA) in adults with obesity, marking a significant milestone in the management of this potentially serious sleep disorder affecting millions worldwide.
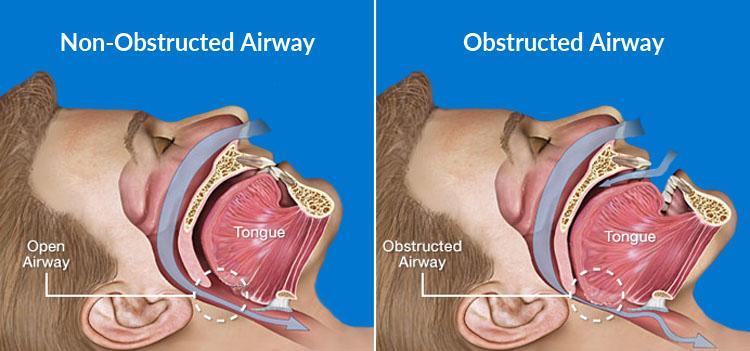
Understanding Obstructive Sleep Apnea
Obstructive Sleep Apnea occurs when the upper airway becomes repeatedly blocked during sleep, leading to pauses in breathing that can last from a few seconds to minutes. These interruptions not only disturb natural sleep patterns but can also lead to serious health complications if left untreated.
Risk Factors and Causes
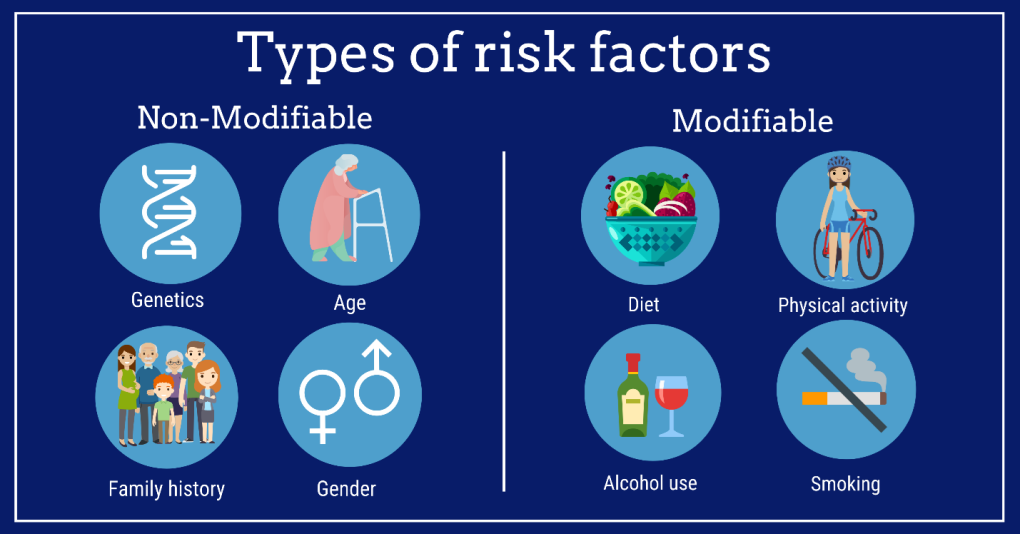
While OSA can affect anyone, certain factors significantly increase the risk:
- Obesity is the primary risk factor, as excess weight around the neck can compress the airway
- Age, particularly middle age and older
- Male gender, though post-menopausal women are also at increased risk
- Family history of sleep apnea
- Smoking and alcohol consumption
- Anatomical features such as a narrow throat or thick neck
Health Implications
The impact of untreated OSA extends far beyond poor sleep quality. Patients may experience:
- Daytime fatigue and difficulty concentrating
- Morning headaches
- High blood pressure
- Increased risk of heart disease and stroke
- Memory problems
- Mood changes and depression
- Complications during surgery and medication use
Revolutionary Treatment Approach
The FDA's approval of Zepbound represents a paradigm shift in OSA treatment. The medication works through a dual mechanism, targeting both GLP-1 and GIP hormones to reduce appetite and food intake. In clinical trials involving 469 adults, participants showed significant improvement in their Apnea Hypopnea Index (AHI) scores after 52 weeks of treatment.
Traditional treatment options include:
- Positive Airway Pressure (PAP) therapy, considered the gold standard
- Oral appliances
- Surgery in select cases
- Lifestyle modifications
Prevention and Management Strategies
While some risk factors for OSA are unchangeable, several preventive measures can reduce the risk or severity:
Lifestyle Modifications
- Weight Management
- Maintaining a healthy weight through proper diet and exercise
- Working with healthcare providers on sustainable weight loss plans
- Regular physical activity
- Sleep Hygiene
- Maintaining regular sleep schedules
- Creating a comfortable sleep environment
- Avoiding alcohol and sedatives before bedtime
- Positional Therapy
- Sleeping on one's side rather than back
- Using specialized pillows or position devices
When to Seek Medical Attention
Individuals should consult healthcare providers if they experience:
- Loud snoring with periods of silence
- Gasping or choking during sleep
- Excessive daytime sleepiness
- Morning headaches
- Difficulty concentrating

Looking Ahead
The approval of Zepbound marks a significant advancement in OSA treatment, particularly for patients who struggle with traditional therapies. However, experts emphasize that it should be used as part of a comprehensive treatment approach that includes lifestyle modifications and regular medical monitoring.
For patients considering Zepbound, it's crucial to discuss potential side effects and contraindications with healthcare providers, as the medication carries important safety considerations, including risks of thyroid tumors and pancreatic inflammation.
This breakthrough in OSA treatment highlights the evolving understanding of sleep disorders and their connection to metabolic health, promising more targeted therapeutic approaches in the future.



